 In
this section we will explore the use of carbon dating to determine the
age of fossil remains.
In
this section we will explore the use of carbon dating to determine the
age of fossil remains.
Carbon is a key element in biologically important
molecules. During the lifetime of an organism, carbon is brought into
the cell from the environment in the form of either carbon dioxide or
carbon-based food molecules such as glucose; then used to build biologically
important molecules such as sugars, proteins, fats, and nucleic acids.
These molecules are subsequently incorporated into the cells and tissues
that make up living things. Therefore, organisms from a single-celled bacteria
to the largest of the dinosaurs leave behind carbon-based remains.
Carbon dating is based upon the decay of 14C, a radioactive
isotope of carbon with a relatively long half-life (5700 years). While 12C
is the most abundant carbon isotope, there is a close to constant
ratio of 12C
to 14C in the environment, and hence in the molecules, cells,
and tissues of living organisms. This constant ratio is maintained until
the death of an organism, when 14C
stops being replenished. At this point, the overall amount of 14C
in the organism begins to decay exponentially. Therefore,
by knowing the amount of
14C in fossil remains, you can determine how long ago
an organism died by examining the departure of the observed 12C
to 14C
ratio from the expected ratio for a living organism.
Decay of radioactive isotopes
 Radioactive
isotopes, such as 14C, decay exponentially. The half-life
of an isotope is defined as the amount of time it takes for there to be
half the initial amount of the radioactive isotope present.
Radioactive
isotopes, such as 14C, decay exponentially. The half-life
of an isotope is defined as the amount of time it takes for there to be
half the initial amount of the radioactive isotope present.
For
example, suppose you have N0 grams of a
radioactive isotope that has a half-life of t* years.
Then we know that after one half-life (or t* years
later), you will have
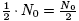 grams
of that isotope.
grams
of that isotope.
t* years after that (i.e. 2t* years from
the initial measurement), there will be
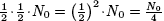 grams.
grams.
3t* years after the initial measurement there will be
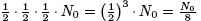 grams,
grams,
and so on.
We can use our our general
model for exponential decay to calculate the amount of carbon at
any given time using the equation,
N (t) = N0e kt
.
Modeling the decay of 14C.
Returning to our example of carbon, knowing that the half-life of 14C
is 5700 years, we can use this to find the constant, k.
That is when t = 5700, there is half the initial amount of 14C.
Of course the initial amount of
14C is the amount of 14C when t =
0, or
N0 (i.e. N(0) = N0e k⋅0 = N0e0 = N0).
Thus, we can write:
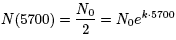 .
.
Simplifying this expression by canceling the N0 on
both sides of the equation gives,
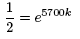 .
.
Solving for the unknown, k, we take the natural logarithm of both
sides,
![equation 1 ln(1/2) = ln e^5700k = 5700k; equation 2 k=[ln(1/2)]/5700 = -0.0001216](graphics/double_eq.gif) .
.
Thus, our equation for modeling the decay of 14C is given by,
 .
.
Other radioactive isotopes are also
used to date fossils.
The half-life for 14C is
approximately 5700 years, therefore the 14C isotope is only useful
for dating fossils up to about 50,000 years old. Fossils older than 50,000
years may have an undetectable amount of 14C. For older
fossils, an isotope with a longer half-life should be used. For example,
the radioactive isotope potassium-40 decays to argon-40
with a half life of 1.3 billion years. Other isotopes commonly used for
dating include uranium-238 (half-life of 4.5 billion years) and thorium-232
(half-life 14.1 billion years).
*****
 In
this section we will explore the use of carbon dating to determine the
age of fossil remains.
In
this section we will explore the use of carbon dating to determine the
age of fossil remains.  Radioactive
isotopes, such as 14C, decay exponentially. The half-life
of an isotope is defined as the amount of time it takes for there to be
half the initial amount of the radioactive isotope present.
Radioactive
isotopes, such as 14C, decay exponentially. The half-life
of an isotope is defined as the amount of time it takes for there to be
half the initial amount of the radioactive isotope present. ![equation 1 ln(1/2) = ln e^5700k = 5700k; equation 2 k=[ln(1/2)]/5700 = -0.0001216](graphics/double_eq.gif) .
.