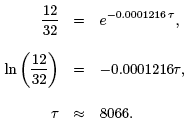| Use the equation we have derived for carbon dating, N(t) = N0 e − 0.0001216 t,
to answer the following question. It may be helpful to know that the
half-life of 14C is 5700 years. |
||
Problem 2- Calculate the age of a fossil Approximately how old is a fossil with 12 g of 14C if it initially possessed 32 g of 14C? |
||
Tutorial Using our model with N0 = 32, N(t) = 32 e − 0.0001216 t. We would like to know how long it takes for the initial 32 g of 14C to decay to 12 g. Let’s call the time it takes for this decay to occur, τ. Then we write, N(τ) = 12 = 32 e − 0.0001216τ . Solving for τ,
Again, this makes sense in light of the half-life. That is, 16 g of 14C would remain after 5700 years and 8 g would remain after 11,400 years.
|
||